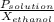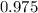Chemistry, 10.08.2019 04:10 brittneys55
What is the vapor pressure of the pure solvent if the vapor pressure of a solution of 10 g of sucrose (c6h12o6) in 100 g of ethanol (c2h6o) is 55 mmhg?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

You know the right answer?
What is the vapor pressure of the pure solvent if the vapor pressure of a solution of 10 g of sucros...
Questions











Biology, 13.08.2020 21:01


Mathematics, 13.08.2020 21:01


Health, 13.08.2020 21:01




History, 13.08.2020 21:01

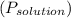 = 55 mm Hg
= 55 mm Hg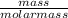
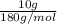
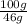
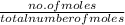
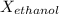 =
= 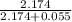
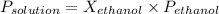
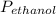 =
=