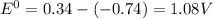Agalvanic (voltaic) cell consists of an electrode composed of chromium in a 1.0 m chromium(iii) ion solution and another electrode composed of copper in a 1.0 m copper(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25 °c. refer to the list

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of chromium in a 1.0 m chromium(iii) ion...
Questions


Mathematics, 11.03.2020 21:46












History, 11.03.2020 21:47

Social Studies, 11.03.2020 21:47


History, 11.03.2020 21:47



Mathematics, 11.03.2020 21:47

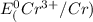 =-0.74V[/tex]
=-0.74V[/tex]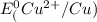 =0.34V[/tex]
=0.34V[/tex]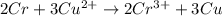
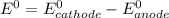
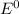 are standard reduction potentials, when concentration is 1M.
are standard reduction potentials, when concentration is 1M.![E^0=E^0_{[Cu^{2+}/Ni]}- E^0_{[Cr^{3+}/Cr]}](/tpl/images/0174/1025/838bc.png)