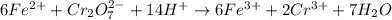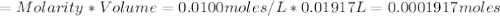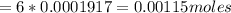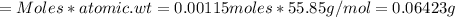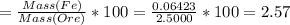Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid solution. a 2.5000-g sample of iron ore is dissolved and the iron converted into iron(ii). exactly 19.17 ml of 0.0100 m na2cr2o7 is required in the titration. what percentage of the ore sample was iron?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid s...
Questions

Chemistry, 19.01.2021 20:30





Mathematics, 19.01.2021 20:30




Mathematics, 19.01.2021 20:30



English, 19.01.2021 20:30

Mathematics, 19.01.2021 20:30

Geography, 19.01.2021 20:30

Mathematics, 19.01.2021 20:30

Mathematics, 19.01.2021 20:30