
Chemistry, 10.08.2019 06:10 lourdess505
Predict the best choice in each of the following. you may wish to review the chapter on electronic structure for relevant examples.
(a) the most metallic of the elements al, be, and ba
(b) the most covalent of the compounds nacl, cacl2, and becl2
(c) the lowest first ionization energy among the elements rb, k, and li
(d) the smallest among al, al+, and al3+
(e) the largest among cs+, ba2+, and xe

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

You know the right answer?
Predict the best choice in each of the following. you may wish to review the chapter on electronic s...
Questions

History, 20.12.2019 01:31



Geography, 20.12.2019 01:31




Chemistry, 20.12.2019 01:31




Spanish, 20.12.2019 01:31


Chemistry, 20.12.2019 01:31

Social Studies, 20.12.2019 01:31

History, 20.12.2019 01:31

Mathematics, 20.12.2019 01:31



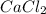 are ionic in nature. On the other hand, in
are ionic in nature. On the other hand, in 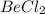 size of Be is smaller and it has high charge density due to which it will attract the valence electrons of chlorine atom.
size of Be is smaller and it has high charge density due to which it will attract the valence electrons of chlorine atom.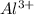 is the smallest.
is the smallest.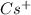 is largest as compared to both
is largest as compared to both 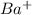 and Xe.
and Xe.

