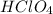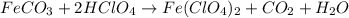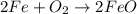Chemistry, 10.08.2019 06:10 briansalazar17
Predict the products of each of the following reactions and then balance the chemical equations.
(a) fe is heated in an atmosphere of steam.
(b) naoh is added to a solution of fe(no3)3.
(c) feso4 is added to an acidic solution of kmno4.
(d) fe is added to a dilute solution of h2so4.
(e) a solution of fe(no3)2 and hno3 is allowed to stand in air.
(f) feco3 is added to a solution of hclo4.
(g) fe is heated in air.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Predict the products of each of the following reactions and then balance the chemical equations.
Questions

Mathematics, 06.05.2020 04:39




History, 06.05.2020 04:39

Spanish, 06.05.2020 04:39

History, 06.05.2020 04:39

Mathematics, 06.05.2020 04:39











Chemistry, 06.05.2020 04:39

Social Studies, 06.05.2020 04:39

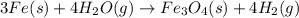
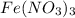
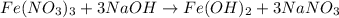
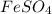 is added to an acidic solution of
is added to an acidic solution of 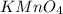
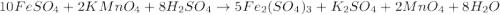
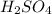
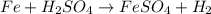
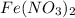 and
and 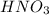 is allowed to stand in air.
is allowed to stand in air.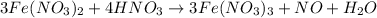
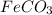 is added to a solution of
is added to a solution of