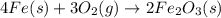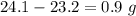Chemistry, 12.08.2019 16:20 novarosell
When elemental iron corrodes it combines with oxygen in the air to ultimately form red brown iron (iii) oxide which we call rust. (a) if a shiny ironnail with an initial mass of 23.2 g is weighed after being coated in a layer of rust, would you expect the mass to have increased, decreased, or remained the same? explain. (b) if the mass of the iron nall increases to 24.1 g what mass of oxygen combined with the iron?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2


You know the right answer?
When elemental iron corrodes it combines with oxygen in the air to ultimately form red brown iron (i...
Questions

Chemistry, 10.03.2020 04:44









Computers and Technology, 10.03.2020 04:44










Physics, 10.03.2020 04:44