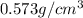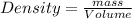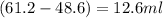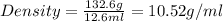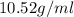Chemistry, 12.08.2019 17:20 jhanley4637
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water. when the jewelry is submerged in the graduated cylinder, the total volume increases to 61.2 ml.
(a) determine the density of this piece of jewelry.
(b) assuming that the jewelry is made from only one suvstance, what substance is it likely to be? explain

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

You know the right answer?
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water...
Questions




Mathematics, 22.01.2020 16:31

Advanced Placement (AP), 22.01.2020 16:31



Health, 22.01.2020 16:31




History, 22.01.2020 16:31





Mathematics, 22.01.2020 16:31


Mathematics, 22.01.2020 16:31