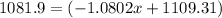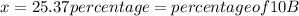The average atomic masses of some elements may vary, depending upon souces of their ores. naturally occurring boron consist of two isotopes with accurately known masses (10 b, 10.0129 amu and 11b, 11.0931 amu). the actual atomic mass of boron can vary grom 10.807 to 10819, depending on whether the mineral source is from turkey or the united states. calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
The average atomic masses of some elements may vary, depending upon souces of their ores. naturally...
Questions


Social Studies, 22.12.2021 21:50

SAT, 22.12.2021 21:50





SAT, 22.12.2021 21:50

Chemistry, 22.12.2021 21:50

Mathematics, 22.12.2021 21:50


Mathematics, 22.12.2021 21:50







Computers and Technology, 22.12.2021 22:00

![\frac{[percentageofisotope(1)Xatomicmassofisotope(1)]+[percentageofisotope(2)Xatomicmassofisotope(2)}{100}](/tpl/images/0174/3972/b6fa0.png)
![10.807=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/4e3aa.png)
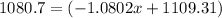
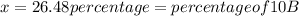
![10.819=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/7b5b5.png)