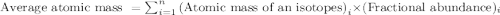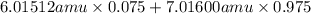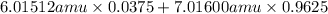Chemistry, 12.08.2019 19:20 kaywendel2008
Variations in average atomic mass may be observed for elements obtained from different sources. lithium provides an example of this. the isotopic composition of lithium from naturally occurring minerals is 7.5% 6li and 92.5% 7li, which have masses of 6.01512 amu and 7.01600 amu, respectively. a commercial source of lithium, recycled from a military source, was 3.75% 6li (and the rest 7li). calculate the average atomic mass values for each of these two sources.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
Variations in average atomic mass may be observed for elements obtained from different sources. lith...
Questions




Mathematics, 10.12.2021 06:40



Mathematics, 10.12.2021 06:40




Mathematics, 10.12.2021 06:40



Mathematics, 10.12.2021 06:40



Mathematics, 10.12.2021 06:50

Mathematics, 10.12.2021 06:50

Mathematics, 10.12.2021 06:50

Chemistry, 10.12.2021 06:50