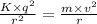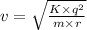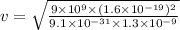Chemistry, 12.08.2019 20:10 vanessaharlan01
Use de broglie's hypothesis to determine the speed of the electron in a hydrogen atom when in the n = 5 orbit, whose radius is 1.3×10−9 m . five de broglie wavelengths should fit around the n = 5 orbit. express your answer with the appropriate units.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Use de broglie's hypothesis to determine the speed of the electron in a hydrogen atom when in the n...
Questions


Arts, 31.07.2019 23:50

Mathematics, 31.07.2019 23:50


Mathematics, 31.07.2019 23:50

Biology, 31.07.2019 23:50


Mathematics, 31.07.2019 23:50


Biology, 31.07.2019 23:50


Mathematics, 31.07.2019 23:50

English, 31.07.2019 23:50


Mathematics, 31.07.2019 23:50

Arts, 31.07.2019 23:50




History, 31.07.2019 23:50