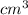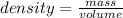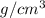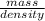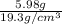Chemistry, 12.08.2019 20:10 Sjanaya853
Given that the density of gold is 19.3 g/cm^3, calculate the volume (in cm^3) of a gold nugget with a mass of 5.98 g. (a) 3.23 cm^3 (b) 5.98 cm^3 (c) 115 cm^3 (d) 0.310 cm^3 (e) 13.3 cm^3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
Given that the density of gold is 19.3 g/cm^3, calculate the volume (in cm^3) of a gold nugget with...
Questions





Spanish, 23.07.2019 16:00


Mathematics, 23.07.2019 16:00

Mathematics, 23.07.2019 16:00



History, 23.07.2019 16:00






Biology, 23.07.2019 16:00

Arts, 23.07.2019 16:00