
Chemistry, 12.08.2019 21:20 edfrank6278
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.500 m solution of aspirin has a ph of 1.86. you are interested in learning about the % dissociation in a buffered solution of aspirin, so you make a new 1.00 l solution containing 0.500 moles of aspirin and 0.25 moles of the sodium salt of aspirin. what will the % dissociation be in this new buffered solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

You know the right answer?
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.50...
Questions

Biology, 14.01.2021 22:50


Mathematics, 14.01.2021 22:50

History, 14.01.2021 22:50




History, 14.01.2021 22:50

Chemistry, 14.01.2021 22:50

Advanced Placement (AP), 14.01.2021 22:50


Physics, 14.01.2021 22:50

History, 14.01.2021 22:50


Mathematics, 14.01.2021 22:50

English, 14.01.2021 22:50

Chemistry, 14.01.2021 22:50

Engineering, 14.01.2021 22:50


![-log[H^{+}]](/tpl/images/0174/4805/1d5a1.png)
![[H^{+}] = 10^{-pH}](/tpl/images/0174/4805/241df.png)
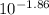
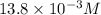
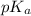 value will be calculated as follows.
value will be calculated as follows.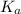 =
= ![\frac{[H^{+}]^{2}}{[Aspirin]}](/tpl/images/0174/4805/0efaa.png)
![\frac{[13.8 \times 10^{-3}]^{2}}{0.50}](/tpl/images/0174/4805/d6d3a.png)
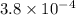
![pK_{a} = -log [K_{a}]](/tpl/images/0174/4805/95c79.png)
![-log [3.8 \times 10^{-4}]](/tpl/images/0174/4805/46d55.png)
![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[Aspirin]}](/tpl/images/0174/4805/cc4ea.png)
![log \frac{[0.25]}{[0.5]}](/tpl/images/0174/4805/52faa.png)
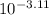
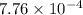 M
M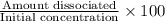
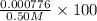
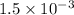 × 100
× 100

