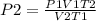Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

You know the right answer?
Consider 4.60 l of a gas at 365 mmhg and 20. ∘c . if the container is compressed to 2.60 l and the t...
Questions

Mathematics, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01







English, 14.10.2020 01:01



Mathematics, 14.10.2020 01:01



History, 14.10.2020 01:01


English, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01