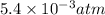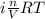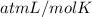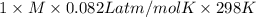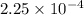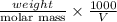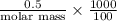Chemistry, 12.08.2019 22:20 cravingnafi202
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and then placed across a semipermeable membrane from a volume of pure water. when the system reaches equilibrium, the solution compartment is elevated 5.6 cm above the solvent compartment. assuming that the density of the solution is 1.0 g / ml, calculate the molecular mass of the unknown.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and...
Questions


History, 18.12.2021 14:00

SAT, 18.12.2021 14:00



Spanish, 18.12.2021 14:10




Social Studies, 18.12.2021 14:10





English, 18.12.2021 14:10





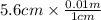 = 0.056, density = 1.0 g/ml, g = 9.8 m/s
= 0.056, density = 1.0 g/ml, g = 9.8 m/s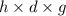
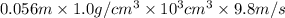
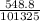 atm
atm