
Chemistry, 12.08.2019 22:30 lovecats12
Consider the following redox equation mn(oh)2(s) + mno4 –(aq) mno42 –(aq) (basic solution) when the equation is balanced with smallest whole number coefficients, what is the coefficient for oh –(aq) and on which side of the equation is oh –(aq) present?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2
You know the right answer?
Consider the following redox equation mn(oh)2(s) + mno4 –(aq) mno42 –(aq) (basic solution) when th...
Questions

Computers and Technology, 12.08.2019 21:30



History, 12.08.2019 21:30


Physics, 12.08.2019 21:30




Mathematics, 12.08.2019 21:30


Social Studies, 12.08.2019 21:30





Social Studies, 12.08.2019 21:30


Biology, 12.08.2019 21:30

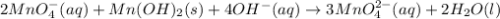
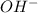 is, 4 and on reactant side of the equation
is, 4 and on reactant side of the equation 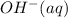 is present.
is present.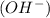 at that side where the less number of hydrogen are present.Now balance the charge.
at that side where the less number of hydrogen are present.Now balance the charge.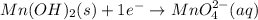 ......(1)
......(1)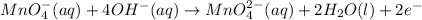 .....(2)
.....(2)

