
A1.0-ml volume of seawater contains about 4.0 x 10^-12 g of gold. the total volume of ocean water is 1.5 x 1021l .calculate the total amount of gold (in grams) that is present in seawater and the worth of the gold in dollars. with so much gold out there, why hasn't someone become rich by mining gold from the ocean?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
A1.0-ml volume of seawater contains about 4.0 x 10^-12 g of gold. the total volume of ocean water is...
Questions

Mathematics, 19.03.2021 14:00

English, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00


Business, 19.03.2021 14:00

French, 19.03.2021 14:00


History, 19.03.2021 14:00



Social Studies, 19.03.2021 14:00




Mathematics, 19.03.2021 14:00

English, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00


Chemistry, 19.03.2021 14:00

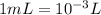
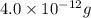 of gold
of gold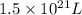 seawater contains
seawater contains 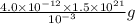 gold or
gold or 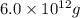 gold
gold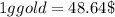
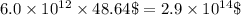
 along with gold in very very small amount.
along with gold in very very small amount.


