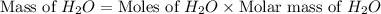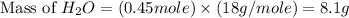Chemistry, 12.08.2019 23:30 nmartin5185
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
ch4 (g) + 2 o2 (g) --> co2 (g) + 2 h2o (l)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 10:30
How is it possible for someone to put an ear to a wall and hear someone in the next room? a.sound waves can travel though solids. b.the waves travel from room to room via air. c.there must be some air in the wall so the sound can travel through it. d.sound waves change to electromagnetic waves and then back again.
Answers: 1
You know the right answer?
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
Questions


Mathematics, 02.02.2021 02:40

Mathematics, 02.02.2021 02:40






Mathematics, 02.02.2021 02:40

Mathematics, 02.02.2021 02:40

Mathematics, 02.02.2021 02:50

German, 02.02.2021 02:50

Medicine, 02.02.2021 02:50



Social Studies, 02.02.2021 02:50


Mathematics, 02.02.2021 02:50


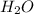 produced will be, 8.1 grams.
produced will be, 8.1 grams.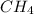 =
= 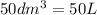
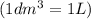
 =
= 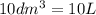
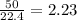 mole of
mole of 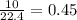 mole of
mole of 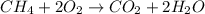
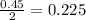 moles of
moles of