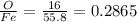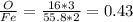Chemistry, 13.08.2019 01:30 katwright1124
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound is given in the following table:
compound mass o : mass fe
feo 0.2865
fe2o3 0.4297
show that these data are consistent with the law of multiple proportions.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

You know the right answer?
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound...
Questions

Mathematics, 08.02.2021 22:40

Social Studies, 08.02.2021 22:40

History, 08.02.2021 22:40


Mathematics, 08.02.2021 22:40


Law, 08.02.2021 22:40

English, 08.02.2021 22:40

Mathematics, 08.02.2021 22:40

English, 08.02.2021 22:40

Mathematics, 08.02.2021 22:40

Mathematics, 08.02.2021 22:40

History, 08.02.2021 22:40






Arts, 08.02.2021 22:40