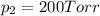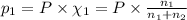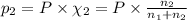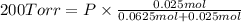Chemistry, 13.08.2019 02:30 lailahussain99
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40.0). if the partial pressure of argon is 200. torr, what is the pressure of methane, in torr? hint: what is the mole fraction of each gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40...
Questions

Physics, 23.02.2021 04:40

Law, 23.02.2021 04:40


Business, 23.02.2021 04:40

History, 23.02.2021 04:40



Mathematics, 23.02.2021 04:40

Mathematics, 23.02.2021 04:40




Spanish, 23.02.2021 04:40


Chemistry, 23.02.2021 04:40


Mathematics, 23.02.2021 04:40



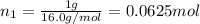
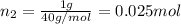
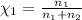
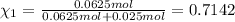
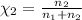
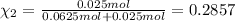
 .
.