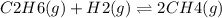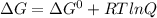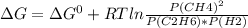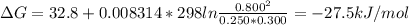For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 atm for all species. for the reaction c2h6(g)+h2(g)↽−−⇀2ch4(g) the standard change in gibbs free energy is δ°=−32.8 kj/mol . what is δg for this reaction at 298 k when the partial pressures are =0.250 atm , =0.300 atm , and =0.800 atm ?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 atm for all specie...
Questions

History, 24.07.2019 01:00

History, 24.07.2019 01:00

Social Studies, 24.07.2019 01:00




Geography, 24.07.2019 01:00

History, 24.07.2019 01:10



Mathematics, 24.07.2019 01:10


Mathematics, 24.07.2019 01:10




Mathematics, 24.07.2019 01:10


English, 24.07.2019 01:10