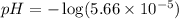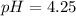Chemistry, 13.08.2019 04:30 jenifferplowman
The acid dissociation ka of propionic acid c2h5co2h is 1.310x10−5. calculate the ph of a 3.010x10−4m aqueous solution of propionic acid. round your answer to 2 decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

You know the right answer?
The acid dissociation ka of propionic acid c2h5co2h is 1.310x10−5. calculate the ph of a 3.010x10−4m...
Questions






Mathematics, 12.08.2020 08:01




English, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01





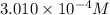
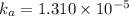
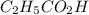 (weak acid) is,
(weak acid) is,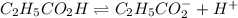
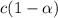
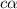
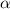 is degree of dissociation
is degree of dissociation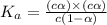
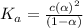
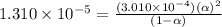
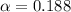
![[H^+]=c\alpha=(3.010\times 10^{-4})\times (0.188)=5.66\times 10^{-5}M](/tpl/images/0174/7681/497f7.png)
![pH=-\log [H^+]](/tpl/images/0174/7681/37e81.png)