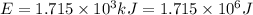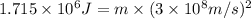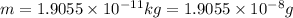In the second footnote it was pointed out that mass and energy are alternate aspects of a single entity called mass-energy. the relationship between these two physical quantities is einstein's equation, e= mc^2, where e is energy, m is mass, and c is the speed of light. in a combustion experiment, it was found that 12.096 g of hydrogen molecules combined with 96.000 g of oxygen molecules to form water and released 1.715 x 10^3 kj of heat. use einstein's equation to calculate the corresponding mass change in this process, and comment on whether or not the law of conservation of mass holds for ordinary chemical processes.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1


Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
In the second footnote it was pointed out that mass and energy are alternate aspects of a single ent...
Questions

Mathematics, 01.06.2022 14:00

Mathematics, 01.06.2022 14:10



Mathematics, 01.06.2022 15:00



English, 01.06.2022 17:10

Chemistry, 01.06.2022 17:10



Biology, 01.06.2022 17:50

Mathematics, 01.06.2022 17:50


English, 01.06.2022 18:20


Mathematics, 01.06.2022 18:30

Business, 01.06.2022 18:40

Chemistry, 01.06.2022 18:50

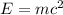 ,
,