
Chemistry, 13.08.2019 05:10 natajaeecarr
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– → h2o + o2 + i– fast which of the following statements is incorrect? a. io– is a catalyst. b. the reaction is first-order with respect to [i–]. c. the reaction is first-order with respect to [h2o2]. d. the net reaction is 2h2o2 → 2h2o + o2. e. i– is a catalyst.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– →...
Questions




















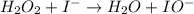 (slow)
(slow)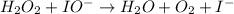
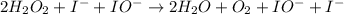
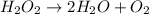
![k[H_{2}O_{2}][I^{-}]](/tpl/images/0174/7990/a32b4.png)
![[I^{-}]](/tpl/images/0174/7990/13772.png) and it is also first order reaction with respect to
and it is also first order reaction with respect to ![[H_{2}O_{2}]](/tpl/images/0174/7990/955b6.png) .
.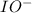 is a catalyst.
is a catalyst.


