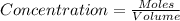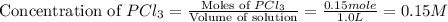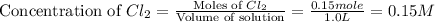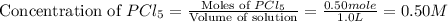Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
Initially, 0.65 mol of pcl5 is placed in a 1.0 l flask. at equilibrium, there is 0.15 mol of pcl3 in...
Questions

Mathematics, 02.05.2021 15:20


Business, 02.05.2021 15:20

Mathematics, 02.05.2021 15:20

Mathematics, 02.05.2021 15:20


Advanced Placement (AP), 02.05.2021 15:20

Mathematics, 02.05.2021 15:20

Mathematics, 02.05.2021 15:20




Mathematics, 02.05.2021 15:20

Chemistry, 02.05.2021 15:20



English, 02.05.2021 15:20



Mathematics, 02.05.2021 15:20

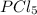 is, 0.50 M
is, 0.50 M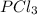 at equilibrium = 0.15 mole
at equilibrium = 0.15 mole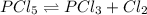
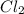 at equilibrium = x = 0.15 mole
at equilibrium = x = 0.15 mole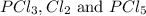 at equilibrium.
at equilibrium.