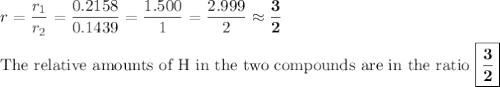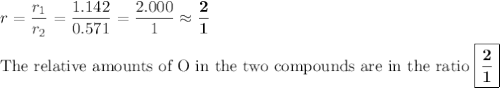Chemistry, 13.08.2019 05:20 quanharris2k19
In each case, calculate the appropriate ratio to show that the information given is consistent with the law of multiple proportions. (a) both ammonia (nh3) and hydrazine (n2h4) are composed of nitrogen and hydrogen. ammonia contains 0.2158 g hydrogen for every gram of nitrogen. hydrazine contains 0.1439 g hydrogen for every gram of nitrogen. (b) two of the compounds that consist of nitrogen and oxygen are nitric oxide, also known as nitrogen monoxide (no) and nitrous oxide (n20), which is also known as dinitrogen monoxide. nitric oxide contains 1.142 g oxygen for every gram of nitrogen. nitrous oxide contains 0.571 g oxygen for every gram of nitrogen.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
In each case, calculate the appropriate ratio to show that the information given is consistent with...
Questions



Physics, 20.05.2020 01:00

History, 20.05.2020 01:00

Chemistry, 20.05.2020 01:00


History, 20.05.2020 01:00

Chemistry, 20.05.2020 01:00



History, 20.05.2020 01:00

Mathematics, 20.05.2020 01:00

Social Studies, 20.05.2020 01:00

Computers and Technology, 20.05.2020 01:00

History, 20.05.2020 01:00

Physics, 20.05.2020 01:00

Mathematics, 20.05.2020 01:00

Mathematics, 20.05.2020 01:00