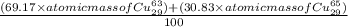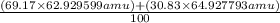Chemistry, 13.08.2019 05:20 johnsonkia873
The atomic masses of the two stable isotopes of copper 63cu29 (69.17 percent) and 65cu29 (30.83 percent)â are 62.929599 and 64.927793 amu, respectively. calculate the average atomic mass of copper.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2


Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
The atomic masses of the two stable isotopes of copper 63cu29 (69.17 percent) and 65cu29 (30.83 perc...
Questions


History, 17.12.2020 18:20


Mathematics, 17.12.2020 18:20


Mathematics, 17.12.2020 18:20


Social Studies, 17.12.2020 18:20






Business, 17.12.2020 18:20



Computers and Technology, 17.12.2020 18:20



Mathematics, 17.12.2020 18:20

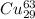 atoms and 30.83 number of
atoms and 30.83 number of 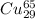 atoms.
atoms.