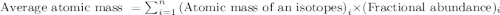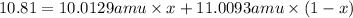Chemistry, 13.08.2019 05:30 luclaymom805
Boron has two naturally occurring isotopes, 10b and 11b, which have masses 10.0129 and 11.0093 amu, respectively. given the average atomic mass of boron (10.81 amu), determine the percent abundance of each isotope. (a) 50%10b, 50%11b (b) 20%10b, 80%11b (c) 98%10b, 2% 11b (d) 93%10b, 7%11b (e) 22%10b, 78%11b

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
Boron has two naturally occurring isotopes, 10b and 11b, which have masses 10.0129 and 11.0093 amu,...
Questions

Mathematics, 12.02.2021 19:40

Mathematics, 12.02.2021 19:40

Mathematics, 12.02.2021 19:40

Mathematics, 12.02.2021 19:40




Chemistry, 12.02.2021 19:40






Chemistry, 12.02.2021 19:40



Mathematics, 12.02.2021 19:40

Mathematics, 12.02.2021 19:40


Mathematics, 12.02.2021 19:40