
Chemistry, 13.08.2019 17:10 mooreadrian4123532
What is the approximate ph at the equivalence point of a weak acid-strong base titration if 25 ml of aqueous formic acid requires 29.80 ml of 0.3567 m naoh? ka =1.8 × 10-4 for formic acid.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

You know the right answer?
What is the approximate ph at the equivalence point of a weak acid-strong base titration if 25 ml of...
Questions




History, 27.01.2020 23:31


History, 27.01.2020 23:31

English, 27.01.2020 23:31





English, 27.01.2020 23:31

English, 27.01.2020 23:31

Computers and Technology, 27.01.2020 23:31



History, 27.01.2020 23:31

Mathematics, 27.01.2020 23:31

History, 27.01.2020 23:31

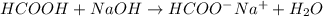
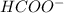
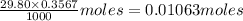
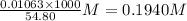
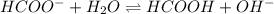
![\frac{[HCOOH][OH^{-}]}{[HCOO^{-}]}=K_{b}(HCOO^{-})=\frac{10^{-14}}{Ka(HCOOH)}](/tpl/images/0174/8862/8150e.png)
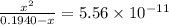
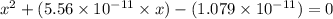
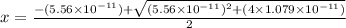
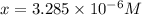
![pH=14-pOH=14+log[OH^{-}]=14+logx=14+log(3.285\times 10^{-6})=8.52](/tpl/images/0174/8862/c94a5.png)


