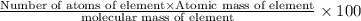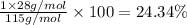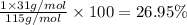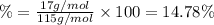Chemistry, 14.08.2019 08:10 anoyinpokep3c3sg
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as a crop fertilizer as well as a component of some fire extinguishers, (a) what are the mass percentages of n and p in the compound? (b) how much ammonia is incorporated into 100. g of compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as...
Questions




SAT, 12.01.2022 14:00

Advanced Placement (AP), 12.01.2022 14:00




Mathematics, 12.01.2022 14:00



Mathematics, 12.01.2022 14:00

Mathematics, 12.01.2022 14:00


Mathematics, 12.01.2022 14:00



Mathematics, 12.01.2022 14:00

Computers and Technology, 12.01.2022 14:00

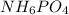 .
.