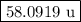Chemistry, 14.08.2019 08:20 petroale000
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu, 932.58%) and 41k osotopic mass 40.9618 amu, 6.730%). fluorine has only one naturally occurring isotope, 19f (isotopic mass 18.9984 amu). calculate the formula mass of potassium fluoride.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 11:30
Which of the following is the most likeley example of an favorable mutation a. a mutation that makes a rabbit able run faster b. a mutation that changes the rabbit's fur to bright orange c. a mutation that changes the color of the rabbit's eyes d. a mutation that gives a rabbit a third ear
Answers: 1
You know the right answer?
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu,...
Questions


English, 04.02.2020 21:52



Mathematics, 04.02.2020 21:52

French, 04.02.2020 21:52

Physics, 04.02.2020 21:52

Mathematics, 04.02.2020 21:52

Mathematics, 04.02.2020 21:52




Health, 04.02.2020 21:52

Mathematics, 04.02.2020 21:52

Social Studies, 04.02.2020 21:52



Social Studies, 04.02.2020 21:52


History, 04.02.2020 21:52