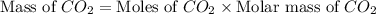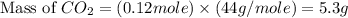Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 1.74 g of butane is mixed with 11. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
A__ is two or more substances that are together in the same place but are not chemically combined
Answers: 1


Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions


Mathematics, 07.09.2021 20:40

Mathematics, 07.09.2021 20:40


Geography, 07.09.2021 20:40

Social Studies, 07.09.2021 20:40


Mathematics, 07.09.2021 20:40



Computers and Technology, 07.09.2021 20:40


History, 07.09.2021 20:40

Social Studies, 07.09.2021 20:40

Biology, 07.09.2021 20:40

Health, 07.09.2021 20:40

History, 07.09.2021 20:40

Mathematics, 07.09.2021 20:40


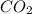 produced will be, 5.3 grams.
produced will be, 5.3 grams.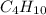 = 1.74 g
= 1.74 g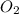 = 11 g
= 11 g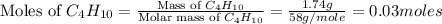
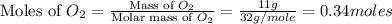
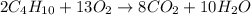
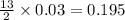 moles of
moles of 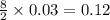 moles of
moles of