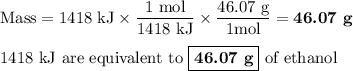Chemistry, 18.08.2019 14:10 rleiphart1
The equation shows one mole of ethanol fuel being burned in oxygen. convert the energy released into its equivalent mass. c2h5oh(l) + 3 o2(g) → 2 co2(g) + 3 h2o (l) δh = -1418 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

You know the right answer?
The equation shows one mole of ethanol fuel being burned in oxygen. convert the energy released into...
Questions

Mathematics, 25.05.2021 20:20

Spanish, 25.05.2021 20:20

Biology, 25.05.2021 20:20




Mathematics, 25.05.2021 20:20


Biology, 25.05.2021 20:20

English, 25.05.2021 20:20

History, 25.05.2021 20:20

Mathematics, 25.05.2021 20:20

Social Studies, 25.05.2021 20:20

Mathematics, 25.05.2021 20:20



Mathematics, 25.05.2021 20:20


English, 25.05.2021 20:20

Spanish, 25.05.2021 20:20