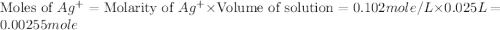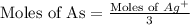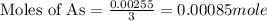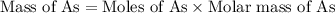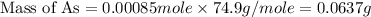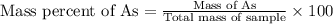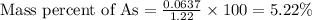Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

You know the right answer?
When a 1.22-g sample of pesticide was analyzed this way, it required 25.0 ml of 0.102 m ag+ solution...
Questions

Chemistry, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57


English, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57



English, 05.06.2020 17:57

Social Studies, 05.06.2020 17:57



English, 05.06.2020 17:57

English, 05.06.2020 17:57





 .
.