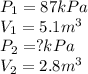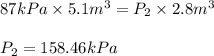Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
If i have 5.1 m3 of gas in a piston at a pressure of 87 kpa and compress the gas until its volume is...
Questions




Chemistry, 16.12.2020 15:50


Mathematics, 16.12.2020 15:50

Mathematics, 16.12.2020 15:50

History, 16.12.2020 15:50





Business, 16.12.2020 15:50




Mathematics, 16.12.2020 15:50

Chemistry, 16.12.2020 15:50

English, 16.12.2020 15:50

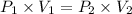
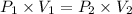
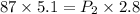
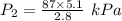
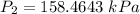
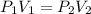
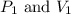 are initial pressure and volume.
are initial pressure and volume.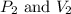 are final pressure and volume.
are final pressure and volume.