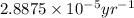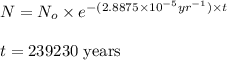Chemistry, 19.08.2019 21:10 ashleyacosta
The spent fuel of a nuclear reactor contains plutonium-239, which has a half-life of 24,000 years. if 1 barrel containing 10 kg of plutonium-239 is sealed, how many years must pass until only 10 g of plutonium-239 is left? use y = y0 *e^ -(kt). (round your answer to the nearest integer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
The spent fuel of a nuclear reactor contains plutonium-239, which has a half-life of 24,000 years. i...
Questions



Mathematics, 09.09.2021 06:00



Mathematics, 09.09.2021 06:00

Mathematics, 09.09.2021 06:00


Mathematics, 09.09.2021 06:00

Mathematics, 09.09.2021 06:00

Social Studies, 09.09.2021 06:00

Mathematics, 09.09.2021 06:00


Engineering, 09.09.2021 06:10


Mathematics, 09.09.2021 06:10

Mathematics, 09.09.2021 06:10

Social Studies, 09.09.2021 06:10


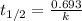
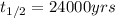
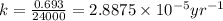
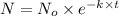
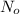 = initial mass of isotope = 10 kg = 10000 g (Conversion factor: 1 kg = 1000 g)
= initial mass of isotope = 10 kg = 10000 g (Conversion factor: 1 kg = 1000 g)