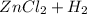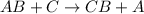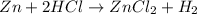Chemistry, 19.08.2019 21:20 KariSupreme
According to the law of conservation of mass, what should the product side of this single replacement reaction look like? a. h2 zncl2 b. h2 + zn + cl2 c. h2 + zncl2 d. zncl2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
According to the law of conservation of mass, what should the product side of this single replacemen...
Questions

Mathematics, 30.06.2021 03:00

Mathematics, 30.06.2021 03:00



Mathematics, 30.06.2021 03:00

Mathematics, 30.06.2021 03:00






Mathematics, 30.06.2021 03:00