
Chemistry, 20.08.2019 00:10 sierralynnbaldp16d4b
Using the standard reduction potentials, pb 2+(aq) + 2e– => pb(s), e° = –0.13 v fe 2+(aq) + 2e– => fe(s), e° = –0.44 v zn 2+(aq) + 2e– => zn(s), e° = –0.76 v which metal will reduce mn 3+ to mn 2+ (e° red = +1.51 v) but will not reduce cr 3+ to cr 2+ (e° red = -0.40 v)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
Using the standard reduction potentials, pb 2+(aq) + 2e– => pb(s), e° = –0.13 v fe 2+(aq) + 2e–...
Questions

History, 09.11.2019 07:31


Mathematics, 09.11.2019 07:31

French, 09.11.2019 07:31

History, 09.11.2019 07:31


Mathematics, 09.11.2019 07:31

Mathematics, 09.11.2019 07:31


Biology, 09.11.2019 07:31







English, 09.11.2019 07:31

English, 09.11.2019 07:31

Social Studies, 09.11.2019 07:31

Arts, 09.11.2019 07:31

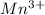 to
to 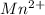
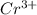 to
to 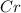
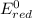 value lower than 1.51 V will donate electron to
value lower than 1.51 V will donate electron to 

