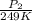The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed in a closed, evacuated container of constant volume at a temperature of 404 k. it is found that all of the ch3i is in the vapor phase and that the pressure is 72.0 mm hg. if the temperature in the container is reduced to 249 k, which of the following statements are correct
choose all that apply
a. the pressure in the container will be 104 mm hg.
b. no condensation will occur.
c. some of the vapor initially present will condense.
d. only pentane vapor will be present.
e. liquid pentane will be present.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

You know the right answer?
The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed i...
Questions









Mathematics, 17.04.2020 04:56











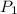 = 72.0 mm Hg,
= 72.0 mm Hg, 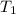 = 404 K
= 404 K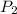 = ? ,
= ? , 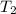 = 249 K
= 249 K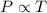 (at constant volume)
(at constant volume) = k
= k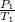 =
= 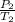
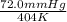 =
=