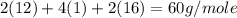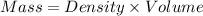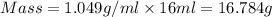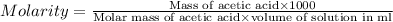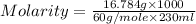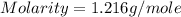Chemistry, 20.08.2019 01:20 anaroles04
Calculate the molarity of a solution of acetic acid made by dissolving 16.00 ml of glacial acetic acid at 25 ∘c in enough water to make 230.0 ml of solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

You know the right answer?
Calculate the molarity of a solution of acetic acid made by dissolving 16.00 ml of glacial acetic ac...
Questions




Social Studies, 01.07.2019 16:30


Mathematics, 01.07.2019 16:30

Mathematics, 01.07.2019 16:30

History, 01.07.2019 16:30


Mathematics, 01.07.2019 16:30






Mathematics, 01.07.2019 16:30

Biology, 01.07.2019 16:30

English, 01.07.2019 16:30

Computers and Technology, 01.07.2019 16:30

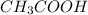 =
=