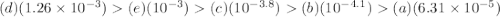Which of the following acids has the weakest conjugate base?
a. benzoic acid, ka = 6.31 x 10...

Chemistry, 20.08.2019 02:30 maddie7417
Which of the following acids has the weakest conjugate base?
a. benzoic acid, ka = 6.31 x 10-5
b. ascorbic acid, pka = 4.1
c. 3-chlorobenzoic acid, pka = 3.8
d. chloroacetic acid, ka = 1.26 x 10-3
e. 2-hydroxybenzoic acid, pka = 3.0

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
Questions


Biology, 01.07.2019 04:40

Physics, 01.07.2019 04:40



Social Studies, 01.07.2019 04:40

Mathematics, 01.07.2019 04:40


Health, 01.07.2019 04:40


Mathematics, 01.07.2019 04:40

History, 01.07.2019 04:40

History, 01.07.2019 04:40


Mathematics, 01.07.2019 04:40


Mathematics, 01.07.2019 04:40

Mathematics, 01.07.2019 04:40

Mathematics, 01.07.2019 04:40

Biology, 01.07.2019 04:40

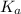 of an acid is a parameter to predict it's acidic strength.
of an acid is a parameter to predict it's acidic strength.