
Chemistry, 20.08.2019 02:30 notseansafe
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium constant for this reaction will
a. increase if the temperature is decreased.
b. decrease with increasing temperature.
c. increase with increasing temperature.
d. increase at some pressures and decrease at other pressures.
e. not change if the temperature is increased.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium con...
Questions




Medicine, 03.12.2021 01:00

Business, 03.12.2021 01:00

Social Studies, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00




English, 03.12.2021 01:00


SAT, 03.12.2021 01:00


Social Studies, 03.12.2021 01:00



Mathematics, 03.12.2021 01:00


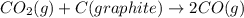
![K_{c} = \frac{[CO_{2}]}{[CO]^{2}}](/tpl/images/0180/4423/2161b.png)


