

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2
You know the right answer?
The complete combustion of octane, c8h18, a component of gasoline, proceeds as follows: 2c8h18(l)+2...
Questions


Biology, 30.01.2020 18:45


History, 30.01.2020 18:45

Physics, 30.01.2020 18:45

Mathematics, 30.01.2020 18:45

Biology, 30.01.2020 18:45

Mathematics, 30.01.2020 18:45

History, 30.01.2020 18:45

Business, 30.01.2020 18:45


Biology, 30.01.2020 18:45

Mathematics, 30.01.2020 18:45

Mathematics, 30.01.2020 18:45

Chemistry, 30.01.2020 18:45




Biology, 30.01.2020 18:45

Mathematics, 30.01.2020 18:45

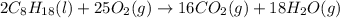
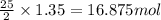 of oxygen are needed.
of oxygen are needed.

