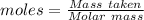Chemistry, 20.08.2019 05:20 catherinesquitieri
What volume, in ml, of a 0.539 m solution of nabh4 is required to produce 0.579 g of b2h6? h2so4 is present in excess.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
What volume, in ml, of a 0.539 m solution of nabh4 is required to produce 0.579 g of b2h6? h2so4 is...
Questions







Mathematics, 30.12.2019 14:31

Biology, 30.12.2019 14:31

Mathematics, 30.12.2019 14:31

Mathematics, 30.12.2019 14:31

Mathematics, 30.12.2019 14:31

Computers and Technology, 30.12.2019 14:31

Mathematics, 30.12.2019 14:31

Mathematics, 30.12.2019 14:31




English, 30.12.2019 14:31

Social Studies, 30.12.2019 14:31