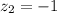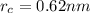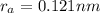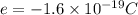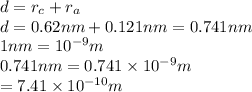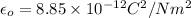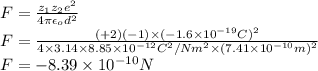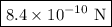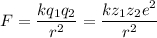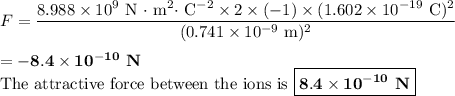Chemistry, 20.08.2019 05:30 JocelynC24
The atomic radii of a divalent cation and a monovalent anion are 0.62 nm and 0.121 nm, respectively. (a) calculate the force of attraction between these two ions at their equilibrium interionic separation (i. e., when the ions just touch one another).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
The atomic radii of a divalent cation and a monovalent anion are 0.62 nm and 0.121 nm, respectively....
Questions

History, 04.11.2021 14:00


Mathematics, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00


Social Studies, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00

Social Studies, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00


Biology, 04.11.2021 14:00


History, 04.11.2021 14:00

Geography, 04.11.2021 14:00

History, 04.11.2021 14:00

History, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00


Social Studies, 04.11.2021 14:00

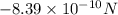 .
.