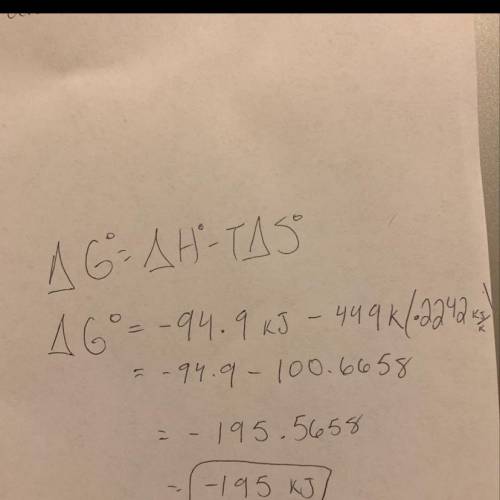Chemistry, 20.08.2019 22:20 anaroles04
Estimate δg°rxn for the following reaction at 449.0 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh°= -94.9 kj; δs°= -224.2 j/k

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Estimate δg°rxn for the following reaction at 449.0 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh°= -94....
Questions






English, 30.10.2020 19:50


Physics, 30.10.2020 19:50


Biology, 30.10.2020 19:50

Mathematics, 30.10.2020 19:50

Mathematics, 30.10.2020 19:50


Spanish, 30.10.2020 19:50



Mathematics, 30.10.2020 19:50


Mathematics, 30.10.2020 19:50

English, 30.10.2020 19:50