
Chemistry, 20.08.2019 22:20 saifulcrc468
Consider the following reaction where kc = 77.5 at 600 k: co(g) + cl2(g) cocl2(g)a reaction mixture was found to contain 2.09×10-2 moles of co(g), 4.17×10-2 moles of cl2(g) and 0.103 moles of cocl2(g), in a 1.00 liter container. indicate true (t) or false (f) for each of the following: 1. in order to reach equilibrium cocl2(g) must be consumed.2. in order to reach equilibrium kc must decrease.3. in order to reach equilibrium co must be produced.4. qc is greater than kc.5. the reaction is at equilibrium. no further reaction will occur.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1
You know the right answer?
Consider the following reaction where kc = 77.5 at 600 k: co(g) + cl2(g) cocl2(g)a reaction mixture...
Questions


Computers and Technology, 19.01.2021 19:40

Mathematics, 19.01.2021 19:40







Geography, 19.01.2021 19:40


History, 19.01.2021 19:40

Mathematics, 19.01.2021 19:40


Mathematics, 19.01.2021 19:40

Mathematics, 19.01.2021 19:40

Mathematics, 19.01.2021 19:40



Mathematics, 19.01.2021 19:40

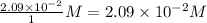
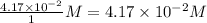
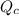 , for this reaction =
, for this reaction = ![\frac{[COCl_{2}]}{[CO][Cl_{2}]}](/tpl/images/0182/8905/92227.png)
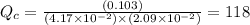
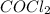 (g) must be consumed and CO must be produced.
(g) must be consumed and CO must be produced.

