
Chemistry, 20.08.2019 23:30 ricardorendon100
Consider the following reaction where kc = 154 at 298 k.2no(g) + br2(g) 2nobr(g)a reaction mixture was found to contain 4.64×10-2 moles of no(g), 4.56×10-2 moles of br2(g) and 0.102 moles of nobr(g), in a 1.00 liter container. is the reaction at equilibrium? if not, what direction must it run in order to reach equilibrium? the reaction quotient, qc, equals .the reactiona. must run in the forward direction to reach equilibrium. b. must run in the reverse direction to reach equilibrium. c. is at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Consider the following reaction where kc = 154 at 298 k.2no(g) + br2(g) 2nobr(g)a reaction mixture w...
Questions

Business, 03.02.2021 14:00

History, 03.02.2021 14:00

Biology, 03.02.2021 14:00

Mathematics, 03.02.2021 14:00

Chemistry, 03.02.2021 14:00

Physics, 03.02.2021 14:00

Engineering, 03.02.2021 14:00

Mathematics, 03.02.2021 14:00

History, 03.02.2021 14:00

History, 03.02.2021 14:00



Social Studies, 03.02.2021 14:00


Arts, 03.02.2021 14:00

Mathematics, 03.02.2021 14:00

Chemistry, 03.02.2021 14:00


Business, 03.02.2021 14:00

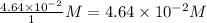
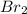 =
= 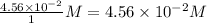
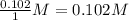
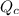 , for this reaction =
, for this reaction = ![\frac{[NOBr]^{2}}{[NO]^{2}[Br_{2}]}](/tpl/images/0183/0904/2c141.png)
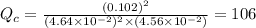
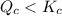 therefore reaction must run in forward direction to increase
therefore reaction must run in forward direction to increase 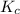 .
.

