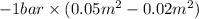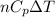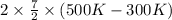Chemistry, 21.08.2019 02:20 BreBreDoeCCx
By heating 2 mol of nitrogen gas in a frictionless piston-cylinder, the gas expands at a constant pres- sure of 1 bar from an initial volume of 0.02 m² to a final volume of 0.05 m. the gas temperature correspondingly increases from 300 to 500 k. assuming nitrogen is an ideal gas with a molar heat capacity cp = r (where r is the gas constant), find the amount of heat added and work derived from the gas expansion.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
By heating 2 mol of nitrogen gas in a frictionless piston-cylinder, the gas expands at a constant pr...
Questions


History, 24.10.2021 21:20




World Languages, 24.10.2021 21:20

Mathematics, 24.10.2021 21:20

Mathematics, 24.10.2021 21:20

Mathematics, 24.10.2021 21:20

Mathematics, 24.10.2021 21:20


Mathematics, 24.10.2021 21:20

Mathematics, 24.10.2021 21:20

Mathematics, 24.10.2021 21:20



Spanish, 24.10.2021 21:20



History, 24.10.2021 21:20

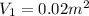 ,
, 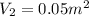
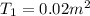 = 300 K ,
= 300 K , 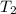 = 500 K
= 500 K