
Asolution is 0.010 m in each of pb(no3)2, mn(no3)2, and zn(no3)2. solid naoh is added until the ph of the solution is 8.50. which of the following statements is true? salt ksp pb(oh)2 1.4 × 10–20 mn(oh)2 2.0 × 10–13 zn(oh)2 2.1 × 10–16 a. all three hydroxides will precipitate. b. no precipitate will form. c. only pb(oh)2 will precipitate. d. only mn(oh)2 will precipitate. e. only zn(oh)2 and pb(oh)2 will precipitate.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Asolution is 0.010 m in each of pb(no3)2, mn(no3)2, and zn(no3)2. solid naoh is added until the ph o...
Questions

Mathematics, 11.04.2020 03:43


Mathematics, 11.04.2020 03:43

English, 11.04.2020 03:43




Chemistry, 11.04.2020 03:43







Mathematics, 11.04.2020 03:44





History, 11.04.2020 03:44

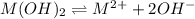
![K_{sp}=[M^{2+}][OH^{-}]^{2}](/tpl/images/0183/5838/c78d3.png)
![[M^{2+}]](/tpl/images/0183/5838/437cc.png) and
and ![[OH^{-}]^{2}](/tpl/images/0183/5838/53c7a.png) exceeds
exceeds 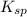 values of given hydroxides then hydroxides of given metal ions will precipitate.
values of given hydroxides then hydroxides of given metal ions will precipitate.![-log[OH^{-}]](/tpl/images/0183/5838/04732.png) = 5.50
= 5.50![[OH^{-}]](/tpl/images/0183/5838/e46dd.png) =
= 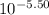
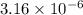
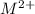 ) are 0.010 M
) are 0.010 M![[M^{2+}][OH^{-}]^{2}=0.010\times (3.16\times 10^{-6})^{2}=3.16\times 10^{-8}](/tpl/images/0183/5838/d1788.png)


