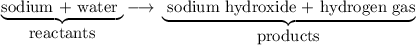Chemistry, 21.08.2019 19:30 silvajimena74
You mix solution a with solution b in a beaker. which of the following observations does not you prove that a chemical reaction has occurred?
a the beaker becomes warm.
b you see gas bubble out of solution.
c solution a, solution b, and the mixture are all yellow.
d none of the above; all prove that a chemical reaction has occurred.
in the reaction described by the word equation sodium + water sodium hydroxide + hydrogen gas
a sodium hydroxide is a product.
b sodium is a product.
c both (a) and (c)
d water is a reactant.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Acycloalkane molecule contains 8 carbon atoms. how many hydrogen atoms are present in the molecule?
Answers: 2


Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
You mix solution a with solution b in a beaker. which of the following observations does not you pr...
Questions

Mathematics, 20.10.2020 22:01

Chemistry, 20.10.2020 22:01

Biology, 20.10.2020 22:01



Mathematics, 20.10.2020 22:01

Health, 20.10.2020 22:01



Mathematics, 20.10.2020 22:01


Chemistry, 20.10.2020 22:01



English, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01




Biology, 20.10.2020 22:01